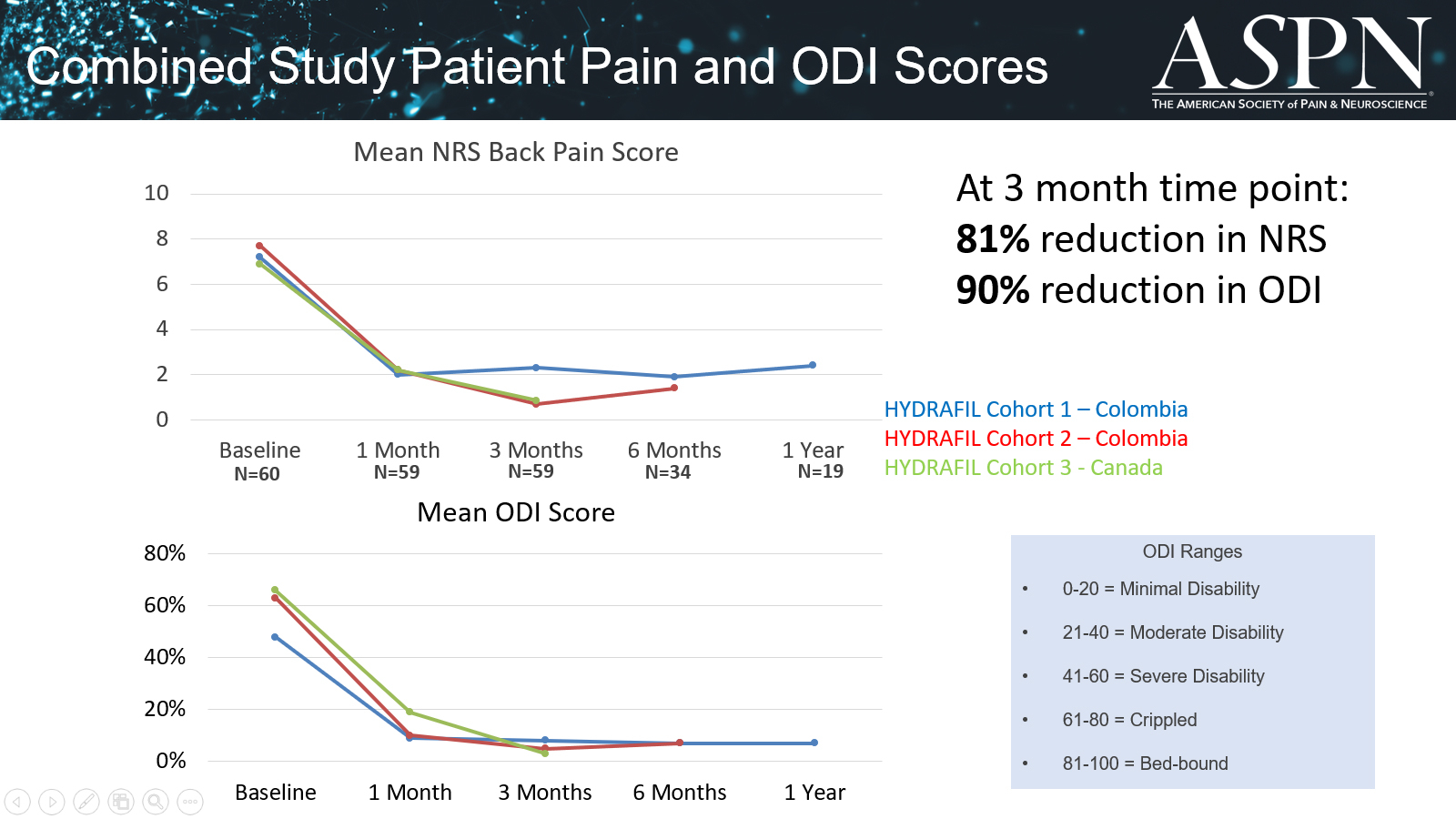
BCWorld Healthcare and ReGelTec have entered into an exclusive distribution agreement for HYDRAFIL™ in the South Korean market
BCWorld Healthcare also made an investment in ReGelTec and the companies are working together to secure regulatory approval in South Korea