Latest News
HYDRAFIL® Presentation Receives Top Abstract Award at the 6th Annual American Society of Pain and Neuroscience Annual Meeting
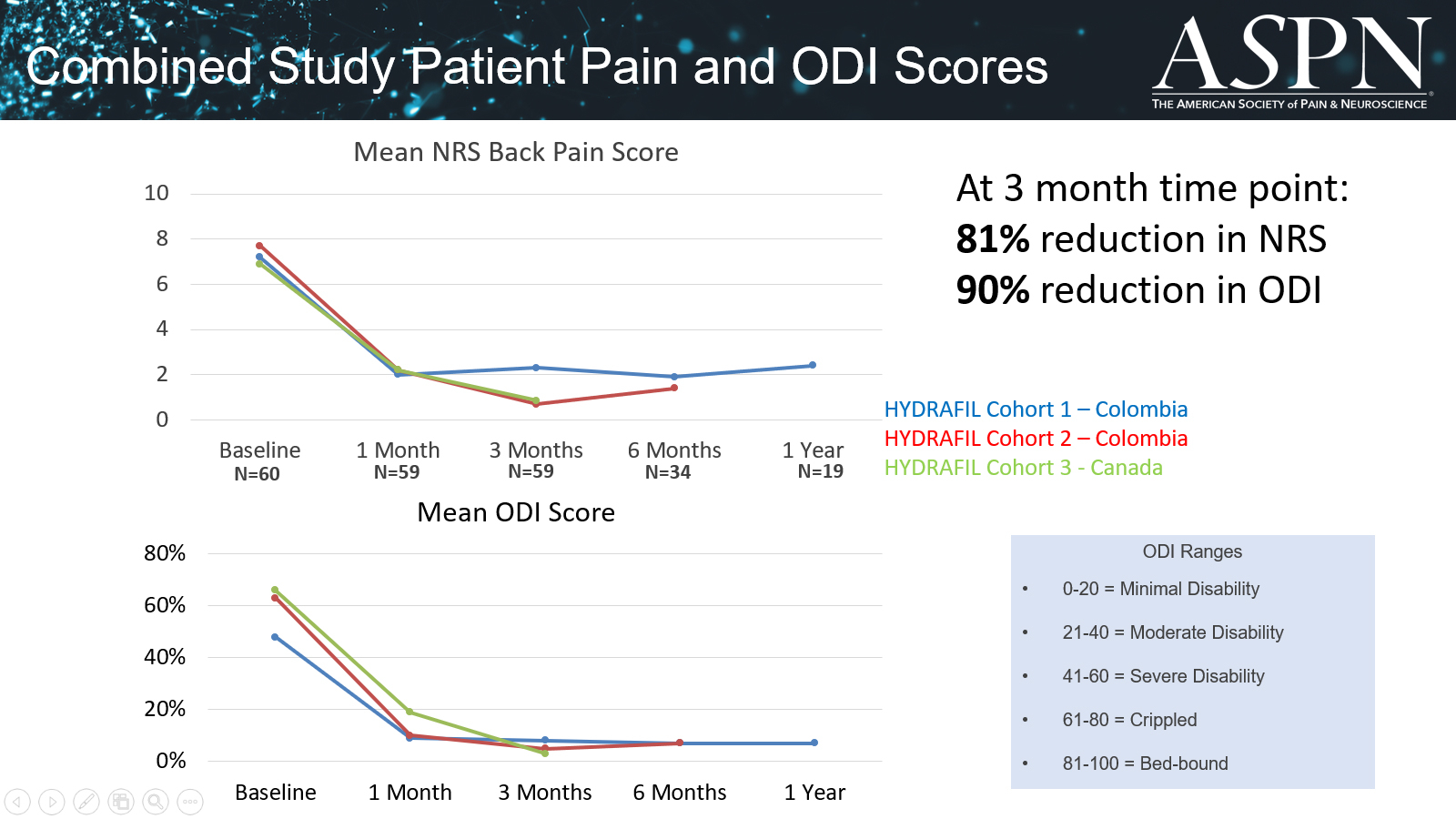
ReGelTec, Inc., announced that Dr. Douglas Beall received the “Top Abstract Award” from the American Society of Pain and Neuroscience (ASPN) for his presentation of the 3-Year follow-up data on chronic low back pain patients treated with ReGelTec’s HYDRAFIL System.
ReGelTec’s Hydrafil Injectable Hydrogel Studied to Treat Low Back Pain Caused by Degenerative Disc Disease
According to the Society of Interventional Radiology (SIR), a small study showed an experimental formulation of a hydrogel injected into spinal discs was safe and effective in substantially relieving chronic low back pain caused by degenerative disc disease (DDD).
The FDA Approves IDE for ReGelTec’s Pivotal Study of HYDRAFIL® for Chronic Low Back Pain due to Degenerative Disc Disease
ReGelTec, Inc., announced that the U.S. Food and Drug Administration has approved an IDE for the company’s pivotal study to support premarket approval of its HYDRAFIL® System.
Injected ‘Hydrogel’ May Be New Option Against Back Pain
Like fixing a flat on the roadside, a new injectable hydrogel is showing promise as a remedy for worn-down spinal discs -- pumping them back up and relieving chronic back pain.
BCWorld Healthcare and ReGelTec have entered into an exclusive distribution agreement for HYDRAFIL™ in the South Korean market
BCWorld Healthcare also made an investment in ReGelTec and the companies are working together to secure regulatory approval in South Korea
ReGelTec’s HYDRAFIL Technology Selected as Best Abstract Presentation
Clinical studies show chronic low back pain decreased by more than 80% in 60 patients at three months.