HYDRAFIL® Presentation Receives Top Abstract Award at the 6th Annual American Society of Pain and Neuroscience Annual Meeting
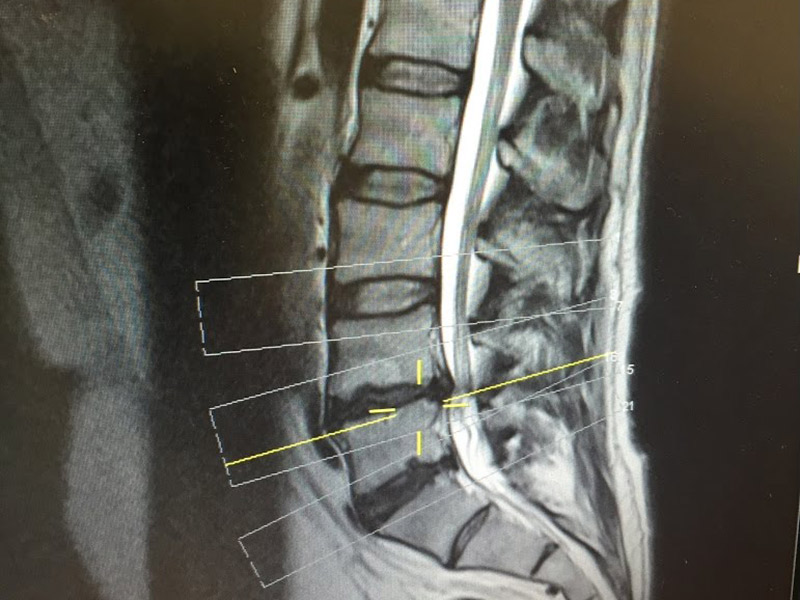
ReGelTec, Inc., announced that Dr. Douglas Beall received the “Top Abstract Award” from the American Society of Pain and Neuroscience (ASPN) for his presentation of the 3-Year follow-up data on chronic low back pain patients treated with ReGelTec’s HYDRAFIL System.