Latest News
ReGelTec Receives FDA Breakthrough Designation for its HYDRAFIL™ System
The Breakthrough Device program is intended to help patients receive faster access to technologies that can provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating conditions.
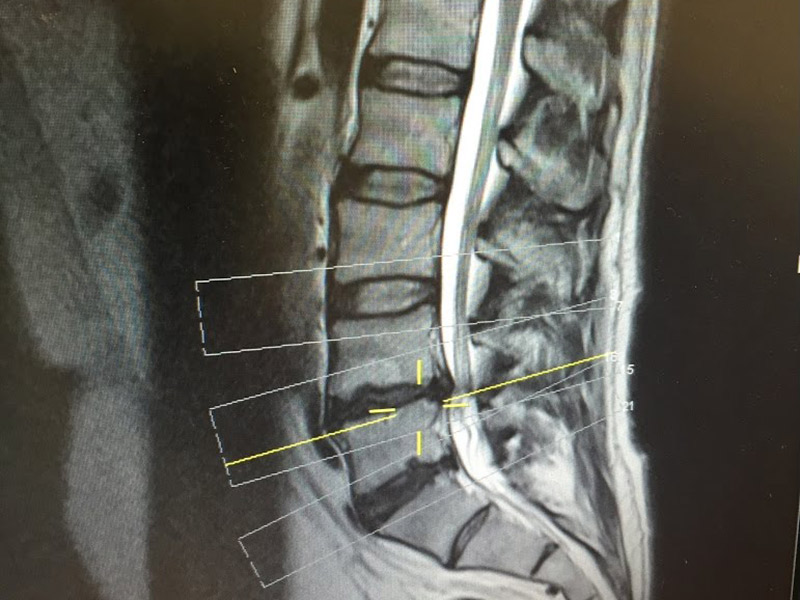
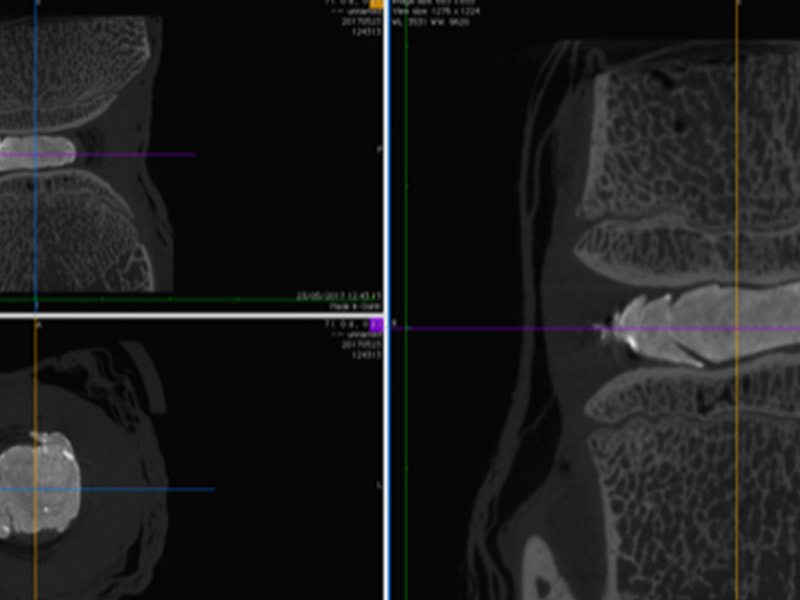
ReGelTec Successfully Treats First Eleven Chronic Low Back Pain Patients with HYDRAFIL™
ReGelTec, Inc., a medical device company developing a percutaneous treatment for chronic low back pain, announced today that eleven patients with degenerative disc disease have been enrolled in the Company’s Early Feasibility Study in Barranquilla, Colombia.
Regeltec chooses Colombia and Bioaccess™ for the first-in-human clinical trial of its HYDRAFIL system
ReGelTec, Inc., a Baltimore-based medical device startup company that is developing the next generation of minimally invasive spinal implants for lower back pain and degenerative disc disease, has chosen Colombia and bioaccess™ as its CRO to conduct the first-in-human clinical trial of its HYDRAFIL System.
ReGelTec, led by former Harpoon Medical executives, raises $2M
CEO Bill Niland and team members behind Harpoon Medical's acquisition are now running a medical device startup seeking to treat degenerative disc disease in the lower back.